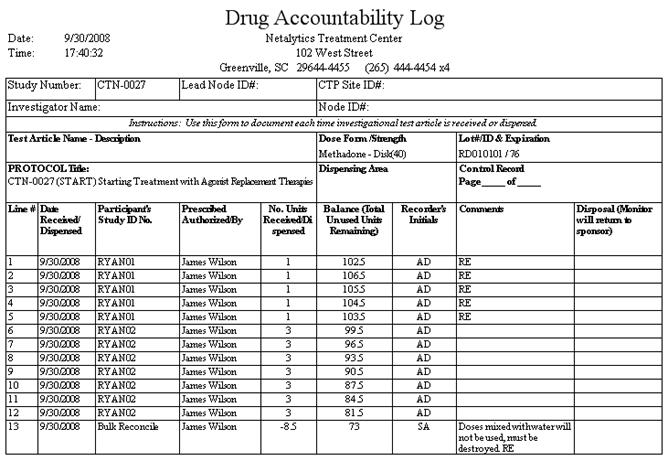
Report: Drug Accountability Log
(Topic Last Updated On: 05/05/2010)
This report is similar to the Bulk Bottle Detail report, displaying all Add Bulk Inventory, Reconcile Bulk Inventory, and Dose Patient transactions entered over a selected date range. This report is grouped by Lot/Bottle Number combination, and will break to a new page for each bottle used. This report has been specifically formatted to meet the needs of specific facilities.

Line #
This column displays the number of each line on this report. Each line presents a dose administered to a patient or an inventory transaction.
Date Received/Dispensed
This column displays the date on which each dose was dispensed or each inventory transaction was recorded in the system.
Participant's Study ID No.
This column displays the 'Patient ID' of the patient who received each dose. 'Bulk Reconcile' appears in this column for reconcile transactions, and 'Receive Bulk' appears in this column for Add Bulk Inventory transactions.
Prescribed/Authorized By
This column displays the full name of the 'Doctor' entered for the 'Home Clinic' on the 'Clinic Information' screen for all transactions.
No. Units Received/Dispensed
This column displays the number of units of medication dispensed, reconciled, or received for each transaction.
Balance (Total Unused Units Remaining)
This column displays a running total of the number of units of medication remaining in the bulk bottle following each transaction.
Recorder's Initials
This column displays the first and last initials of the user who recorded each transaction in the system.
Comments
This column displays the any entered 'Dose Comments' for dosing transactions, 'Additional Bottle Information' for Add Bulk Inventory transactions, and 'Comments' for reconciliation transactions.
Disposal (Monitor will return to sponsor)
This column provides blanks for use in specific facilities and scenarios.
Study Number, Lead Node ID#, CTP Site ID#, Investigator Name, Node ID#, Test Article Name - Description, Protocol Title, Dispensing Area
These fields provide blanks for use in specific facilities and scenarios.
Dose Form/Strength
This field displays the Drug Class and Drug Strength/Form (Drug Type/Dose Type) of the medication contained in the bottle, including the number of mgs contained in one unit of medication.
Lot #/ID & Expiration
This field displays the Lot Number and unique Bulk Barcode ID number of the bottle.
Control Record Page ____ of ____
This field allows for manually documenting which page of how many pages the current page represents.
From Date
This parameter allows users to limit the report's data to only display dosing and inventory transactions entered on or after the date selected here.
To Date
This parameter allows users to limit the report's data to only display dosing and inventory transactions entered on or before the date selected here.
Drug Type
This parameter allows users to limit the report's data to only display dosing and inventory transactions entered for the 'Drug Type' selected here.
Dose Type
This parameter allows users to limit the report's data to only display dosing and inventory transactions entered for the 'Dose Type' selected here.
Dispensed By
This parameter allows users to limit the report's data to only display dosing and inventory transactions entered by the user selected here.
Lot Number
This parameter allows users to limit the report's data to only display dosing and inventory transactions involving bulk bottles with the 'Lot Number' entered here.
Bottle Number
This parameter allows users to limit the report's data to only display dosing and inventory transactions involving bulk bottles with the 'Bottle Number' entered here.